Abstract
Introduction: CDI is common after alloHCT mainly due to the frequent use of antibiotics before and during transplant. CDI is reported in 13 to 18% of recipients after alloHCT and in 6 to 8% after autologous HCT, mainly in the first-month post HCT. The determination of incidence and impact of CDI on HCT outcomes will help further our understanding towards the prevention and management of CDI post HCT.
Methods: Using the CIBMTR dataset, we examined all patients aged two years and older who received first alloHCT for acute myeloid leukemia (AML), Acute Lymphocytic leukemia (ALL), or myelodysplastic syndrome (MDS) from related or unrelated donors between 2013 and 2018 at US centers. Stem cell sources included HLA-matched marrow, peripheral blood (PBSC), and umbilical cord blood (UCB) (4/6 or higher). The objective was to study the impact of CDI by day 100 on transplant outcomes by one year compared to the control cohort (without documented CDI from the same centers). Multivariable analyses were performed using Cox proportional hazard model for overall survival (OS), disease-free survival (DFS), Transplant related mortality (TRM), Infection-related mortality (IRM), chronic GVHD, and relapse. Both aGVHD preceding CDI and CDI preceding aGVHD were analyzed. Due to overlap in the onset of CDI and aGVHD, an interaction of these time-dependent events in some models were noted necessitating incorporation of a composite variable (CV - CDI+aGVHD) for OS, TRM, IRM, and cGVHD models.
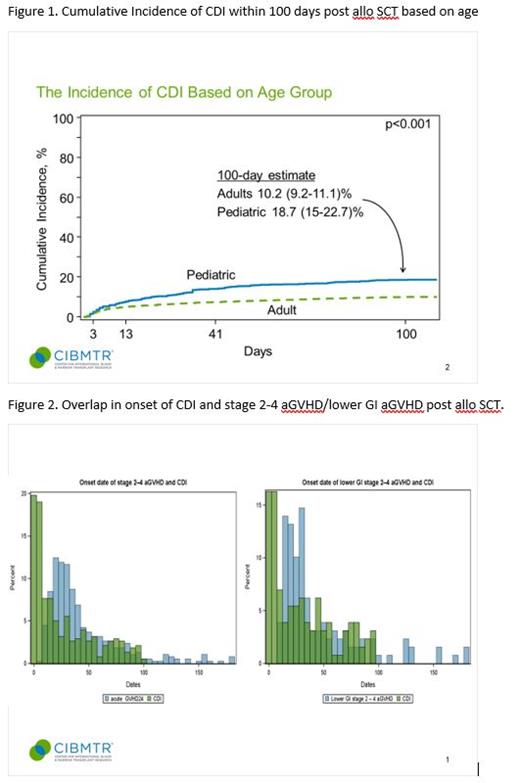
Results: A total of 826 patients with CDI and 6723 controls from 127 centers were analyzed. The cumulative incidence of CDI by day 100 following alloHCT was 18.7% (99% CI: 15% - 22.7%) and 10.2% (99% CI: 9.2% - 11.1%) in pediatric and adult patients, respectively [Figure 1]. The median time to diagnosis of CDI was 13 days (0 - 100). Recurrent CDI by 1 year occurred in 15% of patients. Myeloablative conditioning with total body irradiation (compared to Reduced-intensity/non-myeloablative (RIC/NMA) conditioning) and lower gastrointestinal GVHD preceding the diagnosis of CDI were risk factors for CDI. A diagnosis of MDS was associated with a lower risk of CDI. There was significant overlap in the onset of aGVHD and CDI [Figure 2] such that for patients with both CDI and aGVHD [n=378], 115 (30%) had aGVHD prior to CDI, and 70% were diagnosed with CDI first.
CV - CDI + aGVHD was associated with a statistically significant increase in IRM and TRM and a decrease in OS. Specifically, CDI was associated with a 2.58-fold [99% CI: 1.43 - 4.66; p<0.001] increase in IRM which increased to >4 fold when CV-CDI + aGVHD was considered, irrespective of whether aGVHD preceded CDI or vice versa [CDI first: 4.88 (99% CI: 2.38 - 9.97), p<0.001; aGVHD first 4.15 (99% CI: 1.46 - 11.80), p = 0.0005]. CDI alone was not associated with increased risk of cGVHD [0.85 (99%CI: 0.66-1.09), p=0.0921], and the CV - CDI+aGVHD had similar risk of cGVHD as aGVHD without CDI. CDI had no impact on relapse or DFS.
Conclusion: CDI tightly overlaps with aGVHD. Not surprisingly, the combination of CDI and aGVHD is associated with decreased overall survival and increased TRM. More concerning is that patients having both aGVHD and CDI had a >4-fold increased risk of death due to any infection. Our results highlight the burden and impact of CDI after alloHCT and the critical need to develop new/improved strategies for prevention in alloHCT recipients.
Chemaly: Other: Other: Compensation: I am a consultant and advisor on companies who are developing new agents such as Merck, Ansun, and Janssen. Dandoy: Omeros: Other: Consulted and received Honorarium. Perales: Bristol-Myers Squibb: Honoraria; Sellas Life Sciences: Honoraria; Celgene: Honoraria; Cidara: Honoraria; Equilium: Honoraria; Incyte: Honoraria, Other; Karyopharm: Honoraria; Kite/Gilead: Honoraria, Other; Medigene: Honoraria; Merck: Honoraria; Miltenyi Biotec: Honoraria, Other; MorphoSys: Honoraria; Nektar Therapeutics: Honoraria, Other; NexImmune: Honoraria; Novartis: Honoraria, Other; Omeros: Honoraria; Servier: Honoraria; Takeda: Honoraria. Riches: BioIntelect: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Other: Payment; ATARA Biotherapeutics: Other: Payment. Ustun: novartis: Honoraria; Blueprint: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal